MgBr2 and CaI2 are both ionic solids thus they both have very high melting temperatures due to the strong forces of attraction between ions. Due to the strength of these interactions ionic solids tend to be hard brittle and have high melting points.

Chemical Bonding Doodle Notes Science Doodle Notes Doodle Notes Science Doodle Notes Science Doodles
Identify which ionic solid will have the higher melting temperature.

. And justify your answer. This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point boiling point brittleness solubility and electrical behaviour. Identify which ionic solid will have the higher melting temperature.
1 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. Draw representations of both solids that show the relative differences in the sizes of the four particles and the ratio of. An ion pair A cation and an anion that are in intimate contact in solution rather than separated by solvent.
Learn How to Draw Step by Step in a Fun wayCome join and follow us to learn how Written By leopoldohagos59926 March 26 2022 Add Comment Edit. 1 Draw representations of both ionic solids in each set that show the relative. And justify your answer.
1 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. In order to move forward letti needs to be acreative bopinionated - 6110574. And justify your answer.
Ionic solids are poor conductors of electricity except when their ions are mobile such as when a solid is melted or dissolved in. The net ionic equation is commonly used in acid-base neutralization reactions double displacement reactions and redox reactions. Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion.
Differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. Formula units that contain two ions must be drawn as networks consisting of twelve ions in total. Identify which ionic solid will have the higher melting temperature.
1 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. And justify your answer. Law a higher distance between ionic.
Click here to get an answer to your question lettie is having a problem in her experiment that she does not know how to solve. A large number of ionic solids exhibit one of these five structures which are discussed here. It also explains why caesium chloride has a different structure from sodium chloride even though.
The Fluorite CaF2 Structure 5. Draw representations of both ionic solids. The net ionic equation is a chemical equation for a reaction that lists only those species participating in the reaction.
Formula units that contain two ions must be drawn as networks. Formula units that contain two ions must be drawn as networks consisting. The two sets of ions carry the.
The Sodium Chloride Structure 2. According to Coulombs Law these forces of attraction lattice energy increase as the distance between ionic centres decrease and as the charges on the ions increase. NaCl has a higher melting point because it has a lower atomic radius.
Formula units that contain two ions must be drawn as networks consisting of twelve. Identify which ionic solid will have the higher melting temperature. H of the following isan ionic compound.
In other words the net ionic equation applies to reactions that are strong electrolytes in. The Sodium Chloride Structure. An ion pair can be viewed as a species that is intermediate between the ionic solid and the completely dissociated ions in solution.
Draw representations of both ionic solids in each set that show the relative differences in size of the four ions the ratio of cations to anions and the charge on each ion. These solids are hard and brittle. Identity which ionic solid will have the higher melting temperature and justify your answer.
And justify your answer. In order to move forward l. 7 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion.
Identify which ionic solid will have the higher melting temperature. A unit cell representation of sodium chloride is shown in Fig. Identify which ionic solid will have the higher melting temperature.
Formula units that contain two ions must be drawn as networks. Formula units that contain two ions must be drawn as networks consisting. Ionic solids are composed of cations and anions held together by electrostatic forces.
ACh BN02 COF2 DSCI2 E cot k. And justify your answer. Both ionic of representations.
Formula units that contain two ions must be drawn as networks consisting. Ionic solids typically have high melting points and boiling points. 1 Draw representations of both ionic solids in each set that show the relative.
The Zinc Blende Structure 3. The Wurtzite Structure 4. The Cesium Chloride Structure.
When ionic solids are melted it becomes highly conductive because the molten form of ionic compounds contains ions that. Chemical Bonding MUltiple Choice II Name. Ionic solids containing OH ions are termed as basic compounds because they release OH ions it increase the pH.
Consists of a cation and an anion that are in intimate contact in solution rather than separated by solvent Figure 172 Ion-Pair.

Determining Empirical Molecular Formulas Coloring Activity Molecular Color Activities Covalent Bonding

Solved 7 Draw Representations Of Both Ionic Solids In Each Chegg Com
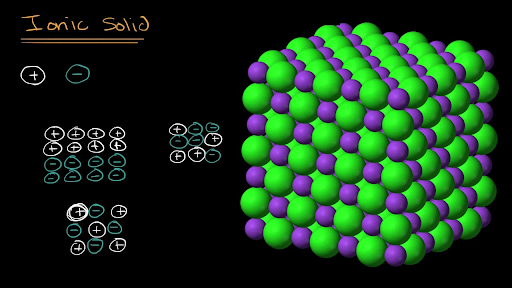
Representing Ionic Solids Using Particulate Models Video Khan Academy
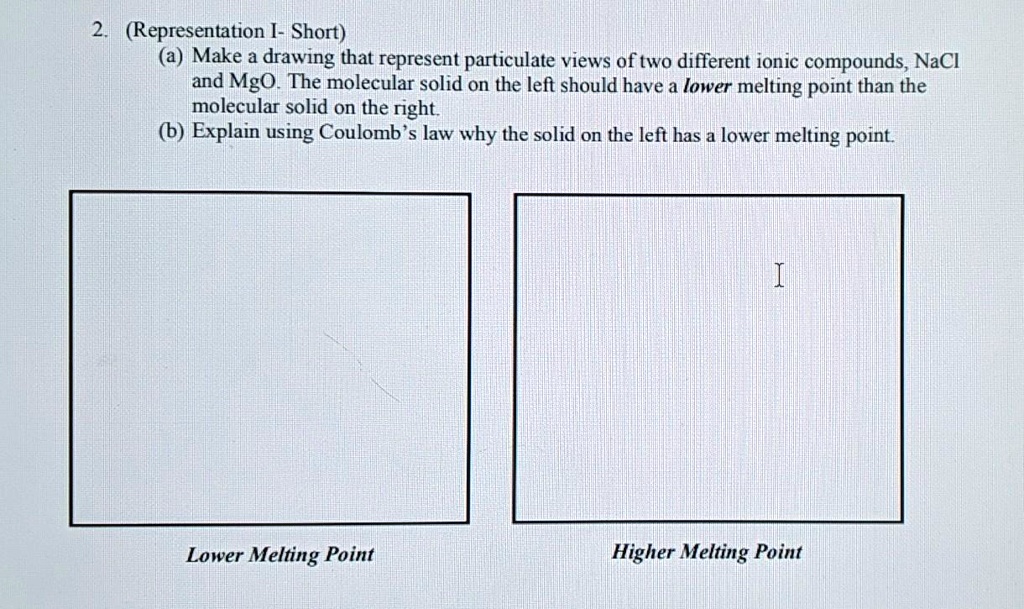
Solved Representation Short A Make A Drawing That Represent Particulate Views Of Two Different Ionic Compounds Nacl And Mgo The Molecular Solid On The Left Should Have A Lower Melting Point Than

Pin By Threefourthsme On Chemistry Covalent Bonding Teaching Style Classwork

How To Draw Lewis Dot Structures For Ionic Compounds Bonds Youtube

2 2 Docx Molecular And Ionic Compound Structure And Properties 2 2 Intramolecular Forces And Potential Energy Worksheet 1 Draw Representations Of Course Hero

Metals Sketch Notes Teaching Chemistry Science Classroom Homeschool Science
0 comments
Post a Comment